- Description
- Type 1 diabetes mellitus
- Guidelines for management of diabetics on sick days
- Diabetes mellitus, insulin dependent: acute complications
- Cerebral oedema in diabetic ketoacidosis
- Diabetic ketoacidosis
- Hypoglycaemia in diabetics
- Diabetic nephropathy
- Dyslipidaemia
- Diabetes mellitus in adolescents
- Diabetes mellitus, type 2
DESCRIPTION
A syndrome of abnormal carbohydrate metabolism, associated with a relative or absolute impairment of insulin secretion with varying degrees of peripheral resistance to the action of insulin.
TYPE 1 DIABETES MELLITUS
E10
DESCRIPTION
Most diabetic children have type 1 diabetes, and:
- have auto-immune destruction of the pancreatic beta cells as the underlying cause,
- have an absolute requirement for insulin therapy,
- will develop diabetic ketoacidosis (DKA) if not given insulin.
DIAGNOSTIC CRITERIA
The following are criteria for the diagnosis of diabetes mellitus:
- Classical features of diabetes (polydipsia, polyphagia, polyuria, weight loss or failure to gain weight, weakness or tiredness, glycosuria, recurrent protracted infections, pruritis vulvae in a girl) with a random serum glucose concentration ≥11.1 mmol/L; or
- Fasting plasma glucose ≥7.0 mmol/L (fasting defined as no caloric intake for at least 8 h);
- An oral glucose tolerance test is generally not needed.
GENERAL AND SUPPORTIVE MEASURES
- Refer to a unit that is able to manage type 1 diabetic patients.
- Educate child and caregiver about all aspects of the disease.
- Medical alert bracelet should be worn at all times.
- Follow up by medical practitioner or at clinic/hospital at least every 3 months.
- Monitor thyroid function annually.
- Screen for coeliac disease at diagnosis, and 3 years post diagnosis.
- Annual screening for dyslipidaemia, microalbuminuria, retinopathy and peripheral neuropathy 5 years after diagnosis in non-pubertal children and 2 years after diagnosis in pubertal children.
Diet: healthy lifelong eating habits
- Refer a newly diagnosed patient and family to a dietician.
- Principles of the prudent diet:
- Encourage children to reduce the intake of fats and salt and to increase dietary fibre content.
- Provide all diabetics with a meal plan, e.g. “constant carbohydrate meal plan” or “carbohydrates counting meal plan”. There is no one ‘diabetic’ diet. Individualise the diet giving consideration to usual eating habits and other lifestyle changes required.
- Six main nutrition factors contribute to better glucose control, i.e. lower HbA1c levels. These are:
- Following a meal plan. Keep day-to-day intake consistent.
- Avoiding extra snacks that are not part of the meal plan.
- Avoiding over-treatment of low blood glucose levels (hypoglycaemia).
- Prompt correction of high blood glucose levels.
- Adjusting insulin levels for meals in patients using the “carbohydrates counting meal plan”.
- Consistency of night snacks.
CONSTANT CARBOHYDRATE MEAL PLAN
Consistency is the key. The amount of insulin, usually two or three doses per day, is kept relatively constant from day-to-day. Carbohydrates should be manipulated to match the relatively constant insulin dose. If able to count carbohydrates, give 1 unit of insulin per 15 g of carbohydrate.
The amount of carbohydrate (types can vary) is kept about the same for each meal and each snack from one day to the next.
As part of the educational process, the family must get used to reading food labels to know the grams (g) of carbohydrates being eaten. The dietician may suggest a range of carbohydrates for each meal.
Examples of carbohydrate content of some foods
The following foods have 15 g of carbohydrate per serving:
1 cup = 250 mL
| FOOD | SERVING SIZE |
|---|---|
| Beans (cooked, canned) | ½ cup |
| Bread (white, brown) | 1 slice |
| Pap (cooked) | ¼ cup |
| Soft maize porridge (cooked) | ½ cup |
| Pasta (cooked) | ½ cup |
| Potato (mashed) | ½ cup |
| Rice (cooked) | ⅓ cup |
| Apple (small) | 1 |
| Fruit juice | ½ cup |
| Grapes | ½ cup (12 medium grapes) |
| Orange (small) | 1 |
| Banana (small) | 1 |
| Milk | 1 cup |
| Yoghurt (low fat, unsweetened) | 1 cup |
| Pizza (thin-crust, medium size) | 1/8 of medium pizza |
| Potato slap chips (not crisps) | 8–12 |
- Tailor the advice to the patients’ lifestyle, economic circumstances and usual diet and, where possible, avoid drastic changes.
- Do not forbid any particular food as this may lead to disturbed attitudes to food, e.g. carbohydrates are not forbidden but can be taken before exercise, incorporated into a main meal or used as a source of energy during illness when children have a poor appetite.
- Diet should provide adequate nutrition for growth and development.
Dietary composition
It is recommended that:
- approximately 35% of dietary energy should be derived from mono- and polyunsaturated fat,
- 15% from protein,
- 50% from carbohydrates. Carbohydrates should always provide at least 40% of the total calories.
Timing of meals and snacks
Children receiving twice daily injections of combined short and intermediate acting insulin regimens need three main meals and three snacks (mid morning, mid afternoon and prior to bed time).
Eat meals and snacks at the same time each day. The timing of insulin injections may need to be adjusted according to the patients’ own circumstances.
Preschool aged children may have unpredictable eating habits and may require frequent small meals.
Exercise
- Regular exercise helps increase insulin sensitivity; maintains proper weight, blood pressure, blood glucose and blood lipid levels.
- Exercise must be regular, i.e. daily. The same amount of exercise should ideally be done at the same time of the day.
- Some form of carbohydrate is necessary before and after intense exercise to reduce the risk of hypoglycaemia. Blood glucose monitoring may be necessary before and after intense exercise.
Blood glucose testing, record keeping and review of records
- Glucometers with compatible strips and bloodletting devices.
- Encourage children to perform their own finger-prick blood glucose testing.
- Finger prick should be performed at the side of the fingertips.
- Encourage the child to monitor his/her blood glucose prior to each main meal and at bedtime. A daily record of all tests performed should be recorded in a logbook. Review logbook frequently to ensure optimal insulin adjustments.
- More frequent blood glucose testing is indicated if the child is unwell, partaking in unusual amounts of physical activity or feels hypoglycaemic.
- For a basal-bolus regimen, testing can be done up to 6 times a day (180 strips/month) and for other regimens, two to four times daily (60 - 120 strips/month). If control is poor, more frequent testing is recommended with appropriate adjustment to therapy.
Glycaemic targets
- Glycaemic targets for young children should not be as strict as for adults. Balance the ability of the family to avoid recurrent hypoglycaemia. A paediatrician should assist in setting practical goals. See table “Monitoring, control and adjustments”.
- Severe hypoglycaemia is the presence of recurrent and unpredictable hypoglycaemic episodes, requiring third party assistance. It leads to anxiety about repeated episodes and results in a poorer quality of life.
- Ideally 80% of the pre-meal blood glucose values should fall within the target range during home monitoring, but targets may need to be altered based on the age of the child and the ability of the family.
- Infants, toddlers, and preschoolers are unable to recognise or communicate signs and symptoms of low blood glucose. They also have unpredictable eating habits.
- Some school-age children and young adolescents have more predictable eating habits, but may be lacking in judgement. They are able to recognise or communicate signs and symptoms of low blood glucose.
- Most adolescents and young adults are able to recognise and treat low blood glucose reactions. They have predictable eating habits and are able to plan ahead.
- Acceptable target range before meals:
| Blood glucose levels | |
|---|---|
| > Infants and toddlers | 6– 12 mmol/L |
| > School-age children and some young adolescents | 4– 10 mmol/L |
| > Most adolescents and young adults | 4– 8 mmol/L |
- Monitor HbA1c levels 3 monthly. The aim is to maintain HbA1C as close as possible to the recommended range, i.e. 6.5 - 7.5%. Aim for a lower HbA1C in patients who are adherent with regard to home glucose monitoring.
Monitoring control and adjustments
|
Level of control |
Optimal |
Suboptimal: (need to take action) |
High risk (refer patient to specialised diabetic clinic) |
|---|---|---|---|
| Clinical assessment | |||
|
Raised blood glucose |
No symptoms |
» polyuria, * » polydypsia, * and » enuresis. * |
» blurred vision, » poor weight gain, » poor growth, » delayed puberty, » poor school attendance, » skin or genital infections, » signs of vascular compromise. |
|
Low blood glucose |
Few, mild No severe hypoglycaemic episodes. |
Severe hypoglycaemia (unconsciousness and/or convulsions) ** |
Monitoring
Biochemical assessment
Self monitoring finger prick glucose monitoring
|
AM fasting (preprandial) |
4-6 | >8 | >9 |
| Postprandial | 5-10 | 10-14 | >14 |
| Bed time | 6.7-10 | <6.7 *** or 10-11 | <4.4 *** or > 11 |
| Nocturnal | 4.5-9 | <4.2 *** or >9 | <4 *** or >11 |
| HbA 1C | 6.5-7.5 | 7.5-9.0 | >9.0 |
*In situations with polyuria, polydypsia and enuresis, adjust the doses of the insulin upwards. Dose adjustments should usually not be greater than 10% of the daily dose at any one time.
** Identify and address the specific reasons for hypoglycaemia e.g. skipping meals or snacks. In specific situations where the lifestyle cannot be modified or there are recurrent episodes of severe hypoglycaemia, consider referral to a tertiary centre.
*** Consider hypoglycaemia unawareness in situations where there are consistently low readings and the patient does not report symptoms.
- Hypoglycaemia unawareness is dangerous. The insulin dose may need to be adjusted downwards if more than 30% of the readings during a single week are below the target values indicated.
Blood or Urine ketone testing
- Hyperglycaemia and a capillary beta-hydroxybutarate level >3 mmol/L indicates that DKA is present. At levels of 0.6-0.3 mmol/L a mild DKA may still be diagnosed.
- If capillary beta-hydroxybutarate strips are not available, significant ketonuria (+++) and hyperglycaemia may also indicate that a DKA is present.
- Test capillary blood or urine for ketones in the following circumstances:
- if vomiting occurs,
- any time the blood glucose >15 mmol/L, especially if the child is unwell and particularly if the blood glucose has been high for more than 24 hours,
- if unusual drowsiness is present,
- in the presence of high temperature, vomiting or diarrhoea, even when the glucose is <15 mmol/L,
- if abdominal pains occur,
- if the breathing is deep and rapid or smells of acetone.
MEDICINE TREATMENT
Insulin therapy
Principles of insulin therapy:
- To provide sufficient insulin throughout the 24-hour period to cover basal requirements.
- To deliver boluses of insulin in an attempt to match the glycaemic effect of meals.
- The most suitable areas for insulin injection are:
- the upper, outer area of the arms;
- the front and side of the thigh;
- the upper, outer surface of the buttocks; and
- the abdomen, except the area close to the navel.
- Establish a pattern for injecting, i.e. horizontally or vertically. Vary the site of injection according to this pattern. When the area has been fully covered move to another area.
- Patients doing strenuous exercise should not inject into their legs.
Insulin injection technique
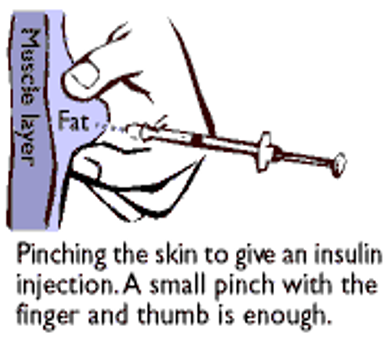
- Insulin injection by syringe is usually given into deep subcutaneous tissue through a two-finger pinch of skin at an angle of 45–90 degrees.
- The subcutaneous fat layer should be thicker than the needle length.
- There is significant risk of accidental intramuscular injections with more rapid absorption, especially in lean individuals. This can be minimised by using a two-finger pinch technique, an injection angle of 90 degrees and use of 5mm needles rather than longer needles in all ages.
- Withdraw the needle and release the skin fold on the count of ten.
- Disinfection of the skin is not necessary prior to insulin injections, however injections should be given through clean, healthy skin.
- Needles should not be used for more than 6 injections.
- Prefilled insulin syringes are recommended for children. Pen devices delivering less than 1 unit should be available for selected patients.
- Thoroughly mix all insulin suspensions before injection by rolling or inverting the vial ten times so that the cloudy suspension mixes thoroughly and uniformly.
Duration of action of standard insulins
| Insulin |
Onset of action |
Peak action |
Effective duration |
|---|---|---|---|
|
Regular/short acting |
30–60 minutes | 2–3 hours | 8–10 hours |
|
Intermediate acting |
2–4 hours | 4–12 hours | 12–20 hours |
Choice of insulin regimen
- No insulin injection regimen satisfactorily mimics normal physiology.
The choice of insulin regimen should be individualised and will depend on age, duration of diabetes, lifestyle (dietary patterns, exercise schedules, school, work commitments, etc), targets of glycaemic control, and particularly, individual patient/family preferences. - The choice of an insulin regimen is determined by the patient’s circumstances. Depending on the patient’s scope to undertake insulin therapy, a number of alternatives will allow insulin therapy to be tailored to their lifestyle. Discussion with parents should provide the basis for such important decisions.
- It is not possible to prescribe a single best regimen for preschool and primary school children. Individualise the choice of regimen according to family circumstances.
- Multiple daily injections provide for the best glycaemic control in young people with type 1 diabetes. If manageable, this should be the regimen of choice. Initially, a twice daily injection regimen may be more manageable.
Questions to be considered when choosing a regimen
What scope does the patient have for insulin therapy?
- Will the patient be able to undertake, financially and culturally, an advanced insulin regimen if necessary?
- Is a responsible person available to give insulin injections at all times of the day or only at certain times?
- How goal-orientated is the patient/caregiver in terms of diabetes control?
What is the patient’s eating pattern?
- What is the typical pattern of meals?
- What type of food do they typically eat at each meal, and how much?
- Is their eating pattern relatively constant, or does it vary?
- Can and will they change their eating habits?
All chosen insulin regimens should be supported by comprehensive education appropriate for the age, maturity and individual needs of the child and family.
Selecting an insulin regimen
Total daily insulin dose
This is individualised and varies according to age, puberty development, stress and individual variability. Usual range is 0.5–1 units/kg/day, but may be higher or lower.
The aim is to select a regimen that allows the achievement of glycaemic control without disabling hypoglycaemia. This also requires a comprehensive support programme for the child and family enabling the implementation of an appropriate diet and other care strategies. These include home blood glucose monitoring and the ability to recognise and manage hypoglycaemic episodes. Where glycaemic control is not achieved despite an adequate support programme consider referral to a tertiary centre.
Insulin regimens
Consult with a paediatric endocrinologist or paediatrician with experience in diabetes care. Repeated consultations are indicated when glycaemic control targets are not achieved.
Basal-bolus regimen
- Short acting insulin 15–30 minutes before a meal or rapid acting insulin with main meals e.g. breakfast, lunch and main evening meal; intermediate acting insulin before bed.
- Normally, 30–40% of the total daily dose of insulin is given at bedtime as intermediate acting insulin. The remaining insulin is given prior to breakfast, lunch and evening meal in the form of short acting insulin.
Basal-bolus regimen
Short acting insulin is indicated in the child (especially <5 years of age) with erratic eating habits despite adequate education
| Breakfast | short acting insulin |
20% of total daily dose (if able to count carbohydrates: give 1 unit per 15 g) |
| Lunch | short acting insulin | 20% of total daily dose |
| Supper | short acting insulin | 20% of total daily dose |
|
At night (± 21h00) |
intermediate acting (ideally this ought to be a basal insulin acting over 24 hours) |
40% of total daily dose |
OR
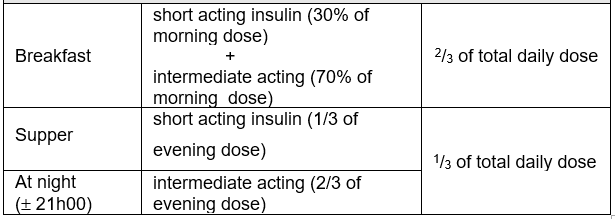
Three injections daily
- A mixture of short and intermediate acting (premixed 70:30) insulin before breakfast; short acting insulin alone before an afternoon snack or main evening meal; intermediate acting insulin before bed; or variations of this regimen may be used at times.
- This requires that the caregiver is aware of three different insulin preparations and can differentiate between them.
Three injections daily
OR
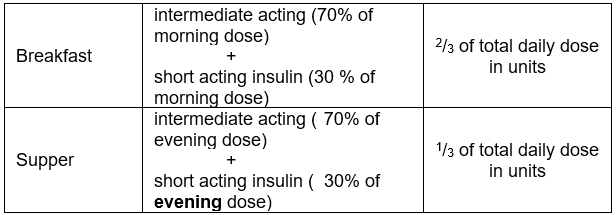
Two Injections daily
- A mixture (premixed combination) of short and intermediate acting insulins (before breakfast and the main evening meal).
- The total daily dose is divided so that 2/3 is given in the morning and 1/3 in the evening.
- The morning or evening dose is then again split between the intermediate-acting and the short-acting insulin in a 70:30 ratio which is pre-mixed
- This regimen is less flexible but easier to instruct.
Two injections daily: Premixed 70/30
None of these regimens can be optimised without frequent assessment of blood glucose monitoring.
Achieving a balance between food intake, insulin levels and energy expenditure is an essential pre-requisite for achieving glycaemic control.
Adjustment of insulin dosage for 3 injection regimen and 2 injection regimen
The insulin dose should not be changed after a single abnormal blood glucose reading.
Adjust the dose only once a pattern has been established. The dose to be adjusted depends on the time of abnormal glucose readings, as indicated in the table below:
|
Timing of the unsatisfactory blood glucose level |
||||
|---|---|---|---|---|
|
Before breakfast |
Before Lunch |
Before supper |
At ± 21h00 | |
| Two injections daily/three injections daily regimens | ||||
|
Insulin dose to be increased if glucose too high _ _ Insulin dose to be decreased if glucose too low |
Supper (in case of premixed insulin) or 21h00 dose: intermediate acting insulin |
Breakfast dose: short acting insulin |
Breakfast dose: intermediate acting insulin |
Supper dose: short acting insulin |
|
Timing of the unsatisfactory blood glucose level |
||||
|
Before breakfast |
Before Lunch |
Before supper |
At ± 21h00 | |
| Basal-bolus regimen | ||||
|
Insulin dose to be increased if glucose too high _ _ Insulin dose to be decreased if glucose too low |
21h00 dose: intermediate acting insulin |
Breakfast dose: rapid (or short acting) insulin |
Lunch dose: rapid (or short acting) insulin |
Supper dose: rapid (or short acting) insulin |
REFERRAL
- Management of all children with diabetes should be supervised by a paediatrician with experience in managing diabetes in the young and should involve a multidisciplinary team, i.e. paediatrician, dietician, nurse educator, psychologist, ophthalmologist.
- Complications.
- Uncontrolled diabetics, such as children with unpredictable blood glucose control, nocturnal or frequent hypoglycaemic events or children who do not reach their therapeutic goals for consideration of analogue insulin.
- Periodic screening of eyes by an ophthalmologist:
- prepubertal onset of diabetes: 5 years after onset and annually thereafter;
- pubertal onset of diabetes: 2 years after onset and annually thereafter.
GUIDELINES FOR MANAGEMENT OF DIABETICS ON SICK DAYS
DESCRIPTION
Illness associated with fever tends to raise blood glucose because of higher levels of stress hormones, gluconeogenesis and insulin resistance.
Illness associated with vomiting and/or diarrhoea may lower blood glucose, with the possibility of hypoglycaemia and the development of starvation ketones.
DIAGNOSTIC CRITERIA
- Unstable blood glucose measurements as a result of illness, stress or starvation.
- Increased insulin requirements are induced by a catabolic state and stress.
- Ketonuria may also indicate the following:
- In the presence of hyperglycaemia, it is indicative of severe insulin deficiency and calls for urgent therapy to prevent progression into ketoacidosis;
- In the presence of low blood glucose levels, it is indicative of a starvation state or is the result of a counter-regulatory response to hypoglycaemia.
GENERAL AND SUPPORTIVE MEASURES
- Monitor glucose more frequently.
- Test urine for ketones.
- Ensure adequate intake of calories and fluids on sick days to prevent ketogenesis. If insufficient calories are consumed, ketones will appear in the urine without hyperglycaemia. In this circumstance encourage the patient to eat whatever he/she feels like.
- Treat underlying intercurrent illness.
- Special circumstances:
- Gastroenteritis:
If hypoglycaemia occurs especially with gastroenteritis, and there is mild ketonuria, ensure that the child takes regular frequent amounts of carbohydrate, using oral rehydration solution or intravenous fluids. - Loss of appetite:
Replace meals with easily digestible food and sugar-containing fluids. - Vomiting:
If the patient has difficulty eating or keeping food down and the blood glucose is <10 mmol/L, encourage the patient to take sugar-containing liquids. Give small volumes. Some glucose will be absorbed. If there is no vomiting, increase the amount of liquid.
- Gastroenteritis:
MEDICINE TREATMENT
Insulin therapy
Insulin must be given every day. Insulin injections should not be omitted because of sickness and/or vomiting. If vomiting occurs, IV fluids may be needed to avoid hypoglycaemia.
During an infection, the daily requirement of insulin may rise by up to 25%.
Generally, the body will require more energy during illness. Insulin allows more glucose to enter the cells, providing more energy to fight infection.
General guidelines when giving extra insulin:
- If the blood glucose is rising or if ketones are present in the urine, the patient must seek urgent medical attention.
Moderate urine ketones
- The extra dose of insulin is usually 10–20% of the total daily dose.
This extra insulin is given as short (or rapid) acting insulin every three hours.
If the blood glucose drops <8.3 mmol/L, it may be necessary to sip regular juice or other sugar-containing drinks. This is done to raise the blood glucose before giving the next insulin injection.
Large amount of urine ketones
- Give 20% of the total daily insulin dose.
Repeat as above if necessary.
Extra fluids
In addition to taking extra insulin, extra fluids, e.g. water and fruit juices are important to prevent acidosis. These fluids replace the fluids lost in the urine and prevent dehydration.
REFERRAL
- In a child with inter-current illness urgent specialist medical or nursing advice must be obtained when:
- patient is unable to carry out the advice regarding sick days;
- the diagnosis is unclear;
- vomiting is persistent, particularly in young children;
- blood glucose continues to rise despite increased insulin;
- hypoglycaemia is severe;
- ketonuria is heavy or persistent;
- the child is becoming exhausted, confused, hyperventilating, dehydrated or has severe abdominal pain.
DIABETES MELLITUS, INSULIN DEPENDENT : ACUTE COMPLICATIONS
E10
CEREBRAL OEDEMA IN DIABETIC KETOACIDOSIS (DKA)
G93.6
DESCRIPTION
A condition of brain swelling during the course of treatment for DKA.
Cerebral oedema usually occurs 4–12 hours after the initiation of treatment, but may be present at the time of diagnosis. It often follows an initial period of clinical and biochemical improvement.
Cerebral oedema causes significant neurological morbidity and has a mortality of approximately 80%.
The cause of cerebral oedema during treatment remains unclear. However, very rapid reduction in intravascular osmolality may aggravate the process. Therefore, rehydration should occur more slowly in children with DKA than in other causes of dehydration.
DIAGNOSTIC CRITERIA
Clinical
- Signs and symptoms of cerebral oedema include:
- headache,
- confusion,
- irritability and restlessness,
- reduced consciousness,
- papilloedema (late sign),
- hypoxaemia, and
- specific neurological signs and raised intracranial pressure.
- The risk of cerebral oedema is increased if urea levels are increased or if the PCO₂ is persistently low, i.e. <20 mmHg.
GENERAL AND SUPPORTIVE MEASURES
- Admit to ICU, if possible, or to a centre experienced with managing this condition.
- Restrict intravenous fluids to ⅔ maintenance and replace deficit over 72 hours rather than 48 hours pending ICU admission.
- Elevate head of bed.
- Exclude hypoglycaemia.
- Do not use bicarbonate.
- Exclude thrombosis, intracranial haemorrhage or infection.
- Do not delay treatment while waiting for a CT scan to confirm cerebral oedema.
MEDICINE TREATMENT
For the management of cerebral oedema, see Status Epilepticus (convulsive) , cerebral oedema.
DIABETIC KETOACIDOSIS
E10.1
DESCRIPTION
Diabetic ketoacidosis (DKA) occurs with relative or absolute insulin deficiency, either caused by non-adherence to insulin regimens or by excessive secretion of counterregulatory hormones during stress, e.g. infection, trauma and surgery.
DIAGNOSTIC CRITERIA
- Heavy glycosuria (2+ or more).
- Hyperglycaemia, i.e. blood glucose usually >11 mmol/L, ketonuria, or/and pH <7.3.
- Bicarbonate <15 mmol/L and patients who are clinically dehydrated.
- May be vomiting.
- May be drowsy.
Note:
In rare cases blood glucose is not elevated.
Children with mild dehydration and not clinically unwell usually tolerate oral rehydration and subcutaneous insulin.
See Guidelines for management of diabetics on sick days .
GENERAL AND SUPPORTIVE MEASURES
- Admit all children and adolescents to an ICU or ward experienced in the management of DKA in children and adolescents, if possible.
- Ensure patent airway.
- If the child is comatose, secure the airway and insert a urinary catheter.
- If comatose or recurrent vomiting insert oro/nasogastric tube and apply free drainage.
MEDICINE TREATMENT
Seek specialist advice early in the management.
If hypoxaemic:
- Oxygen via facemask.
The objectives of fluid and sodium replacement therapy in diabetic ketoacidosis are:
- To restore circulating volume.
- To replace sodium and water deficits from extracellular and intracellular compartments.
- To restore glomerular filtration rate to enhance clearance of glucose and ketones from the blood.
- To reduce the risk of cerebral oedema.
Fluids
a: Fluids for resuscitation in shock:
- Sodium chloride 0.9%, IV, 10–20mL/kg over 10–30 minutes.
- Repeat if shock persists.
b: Fluid requirements after resuscitation
| Calculation of fluid requirement during the subsequent phase of rehydration (see table below for the calculations determined for different weights) |
|---|
| Fluid requirement = deficit + maintenance |
| Calculate deficit = estimated % dehydration x body weight (kg and equivalent in mL) |
|
Calculate maintenance (mL): ≤1 year: 120 mL/kg/24 hours All children older than 1 year – the sum of the following: • first 10 kg body weight: 100 mL/kg/24 hours • second 10 kg body weight: 50 mL/kg/24 hour • additional weight > 20 kg body weight: 20 ml/kg/24 hours |
| Add deficit to 48 hour maintenance and replace this volume evenly over 48 hours, initially with sodium chloride 0.9%. When blood glucose falls to 12–15 mmol/L change the infusion to a dextrose-containing maintenance fluid, e.g. dextrose 5% in sodium chloride 0.45%. |
| Assess hydration status every 4–6 hours |
Examples of fluid volumes for subsequent phase of rehydration (i.e. maintenance + 5% of body weight/24 hours)
|
Body weight kg |
Maintenance mL/24 hour |
Maintenance + 5% of body weight mL/24 hour |
Maintenance + 5% of body weight mL/hour |
|---|---|---|---|
| 4 | 325 | 530 | 22 |
| 5 | 405 | 650 | 27 |
| 6 | 485 | 790 | 33 |
| 7 | 570 | 920 | 38 |
| 8 | 640 | 1040 | 43 |
| 9 | 710 | 1160 | 48 |
| 10 | 780 | 1280 | 53 |
| 11 | 840 | 1390 | 58 |
| 12 | 890 | 1490 | 62 |
| 13 | 940 | 1590 | 66 |
| 14 | 990 | 1690 | 70 |
| 15 | 1030 | 1780 | 74 |
| 16 | 1070 | 1870 | 78 |
| 17 | 1120 | 1970 | 82 |
| 18 | 1150 | 2050 | 85 |
| 19 | 1190 | 2140 | 89 |
| 20 | 1230 | 2230 | 93 |
| 22 | 1300 | 2400 | 100 |
| 24 | 1360 | 2560 | 107 |
| 26 | 1430 | 2730 | 114 |
| 28 | 1490 | 2890 | 120 |
| 30 | 1560 | 3060 | 128 |
| 32 | 1620 | 3220 | 134 |
| 34 | 1680 | 3360 | 140 |
| 36 | 1730 | 3460 | 144 |
| 38 | 1790 | 3580 | 149 |
| 40 | 1850 | 3700 | 154 |
| 45 | 1980 | 3960 | 165 |
| 50 | 2100 | 4200 | 175 |
| 55 | 2210 | 4420 | 184 |
| 60 | 2320 | 4640 | 193 |
| 65 | 2410 | 4820 | 201 |
| 70 | 2500 | 5000 | 208 |
| 75 | 2590 | 5180 | 216 |
| 80 | 2690 | 5380 | 224 |
Note: Sodium chloride 0.9% is preferred for resuscitation and the initial phase of rehydration. However, to prevent the occurrence of hyperchloraemic acidosis switch to sodium chloride 0.45%/dextrose 5% after blood glucose has fallen to 12 mmol/L or less.
Note: One of the danger signals for cerebral oedema is a precipitous drop in the serum sodium level.
Bicarbonate
Bicarbonate use is associated with increased risk of cerebral oedema. It should not be used routinely to improve adicosis.
Caution
Consult a specialist before administering any bicarbonate solution.
Potassium
Commence potassium replacement immediately unless patient has anuria. If serum potassium is start replacement after the patient has passed urine.
Early addition of potassium in the fluid regimen (KCl 15% 20mL in 1L=40mmol/L.) is essential even if the serum concentration is normal as insulin will drive glucose and potassium into the cells.
DKA protocol:
Two-bag system – Alternative fluid and electrolyte treatment
Under supervision of a specialist.
The two-bag system consists of 2 bags of identical electrolyte content but different dextrose concentrations, 0% and 10%, administered simultaneously into a single IV line. Variations in dextrose delivery are achieved through changing the proportions of the 2 bags contributing to the total rate, which is determined by the degree of dehydration.
- Sodium chloride 0.9%, IV, 10–20 mL/kg.
- May be repeated if necessary.
- Then switch to “two bag” system
|
Bag 1 (dextrose 0%) |
Bag 2 (dextrose 10%) |
|---|---|
|
- Sodium chloride 0.45%, 1 L PLUS - Potassium chloride, 20 mL |
- Dextrose 10%, 1 L PLUS - Sodium chloride 5%, 90 mL PLUS - Potassium chloride, 20 mL |
Run these two riders for easy titration of dextrose from dextrose 10% to dextrose 0%:
| Fluid |
Blood glucose >15 |
Blood glucose 10–15 |
Blood glucose <10 |
|---|---|---|---|
| Bag 1 | 100% | 50% | 0% |
| Bag 2 | 0% | 50% | 100% |
Insulin
- Insulin short acting, 0.1unit/kg/hour as a continuous IV infusion.
- Add insulin, 50 units (0.5 mL) to 50 mL sodium chloride 0.9% in a syringe pump to get a solution of 1 unit/mL.
- Attach this using a Y-connector to the IV fluids already being administered.
- Do not add insulin directly to the fluid bags.
- The solution should be administered at a rate of 0.1mL/kg/hour (0.1unit/kg/hour).
If the rate of blood glucose fall exceeds 5mmol/ L/hour or the blood glucose falls to 14 mmol/L:
- Add a dextrose-containing fluid.
- Do not stop the insulin infusion while dextrose is being infused.
If the blood glucose falls below 4 mmol/L:
- Give a bolus of 2 mL/kg of dextrose 10% and increase the concentration of dextrose in the infusion.
Continue with IV insulin until:
- base deficit is < 5 or bicarbonate is 15 mmol/L,
- there is no ketonuria,
- blood glucose is 10mmol/L.
Alternative to insulin infusion
Where there are no facilities for insulin infusion, e.g. no syringe pumps, staff constraints, etc.:
- Insulin short-acting, IV, 0.1 unit/kg, hourly.
Changing from intravenous to subcutaneous insulin
Continue with intravenous fluids until the child is drinking well and able to tolerate snacks. When oral fluids are tolerated, reduce intravenous fluids. Subcutaneous insulin can be started once the child is well hydrated and able to tolerate a normal diet.
The most convenient time to change to subcutaneous insulin is just before a meal. Administer the first dose of subcutaneous insulin 30 minutes before the meal and continue with the insulin infusion for 90 minutes after the subcutaneous injection to prevent rebound hyperglycaemia.
In newly diagnosed diabetics, Basal-Bolus regimen is started as described in Type 1 Diabetes Mellitus – Insulin Regimens , in a low range dose:
- Prepubertal children: 0.7 units/kg.
- Pubertal children: 1 unit/kg.
In established diabetics, give maintenance insulin.
Give supplemental subcutaneous short acting insulin before meals if the blood glucose >11 mmol/L:
|
Blood glucose mmol/L |
Short-acting Insulin units/kg/dose |
|---|---|
| 11-12 | 0.06 |
| 13-16 | 0.09 |
| 16 | 0.12 |
REFERRAL
- No improvement
- Deterioration of condition, i.e.:
- pH <7.1,
- hyperventilation,
- shock,
- depressed level of consciousness,
- persistent vomiting,
- age <5 years.
- Rising blood glucose.
HYPOGLYCAEMIA IN DIABETICS
E16.0
DESCRIPTION
Autonomic symptoms (hunger, nausea, anxiety, pallor, palpitations, sweating, trembling) usually precede neuroglycopaenic symptoms (impaired thinking, change of mood, irritability, dizziness, headache, tiredness, confusion, and later convulsions and coma). Patients with frequent hypoglycaemic episodes develop hypoglycaemia unawareness, where the symptoms above do not occur despite a dangerously low blood sugar level.
Causes of hypoglycaemia include:
- A missed or delayed snack or meal.
- Exercise without appropriate dietary preparation.
- Alcohol.
- Overdose of insulin.
- Impaired food absorption e.g. gastro-enteritis.
- Addison’s disease. Recurrent hypoglycaemia may necessitate investigation for this condition.
- Coeliac disease.
Nocturnal hypoglycaemia
Nightmares and headaches may be suggestive of nocturnal hypoglycaemia.
Blood glucose concentrations fall to their lowest levels between 02h00 and 04h00.
DIAGNOSTIC CRITERIA
- Blood glucose <3.5–4 mmol/L with symptoms in a known diabetic patient.
Good glycaemic control is likely to be associated with occasional hypoglycaemic episodes. - Grading of severity:
- Mild (Grade 1)
- Child or adolescent is aware of, responds to and self-treats the hypoglycaemia.
- Children <6 years of age can rarely be classified as grade 1 because they are unable to help themselves.
- Moderate (Grade 2)
- Child or adolescent cannot respond to hypoglycaemia and requires help from someone else, but oral treatment is successful.
- Severe (Grade 3)
- Child or adolescent is semiconscious or unconscious with or without convulsions and may require parenteral therapy with glucagon or intravenous glucose.
GENERAL AND SUPPORTIVE MEASURES
- Determine underlying cause.
- Patient education on diabetes and its complications.
MEDICINE TREATMENT
Mild or moderate hypoglycaemia:
Immediate oral rapidly absorbed simple carbohydrate, e.g.:
- Glucose, oral, 5–15 g or 1-3 level teaspoons of sugar (depending on child's age) in a small amount of water.
- Wait 10–15 minutes.
- If blood glucose has not risen to 6-8 mmol/L, repeat above.
- As symptoms improve, the next meal or oral complex carbohydrate should be ingested, e.g. fruit, bread, cereal, milk, etc.
Severe hypoglycaemia
Outside hospital
- Glucagon, IM/SC, 0.1–0.2 mg/10 kg body weight.
- If < 12 years of age: 0.5 mg.
- If > 12 years of age: 1.0 mg.
If glucagon is not available:
A teaspoon of sugar moistened with water placed under the tongue, every 20 minutes until patient awakes.
In hospital
If there is an unsatisfactory response or inability to take oral carbohydrate and signs of disorientation, stupor, convulsions, coma:
- Dextrose 10%, IV, 2–5 mL/kg.
- Dilute dextrose 50% solution to 10% strength before use.
- i.e. Dextrose 50% 1 mL + water for injection 4 mL = 5 mL 10% dextrose solution.
If IV dextrose cannot be given:
- Glucagon, IM/SC, 0.1–0.2mg/10kg body weight.
- If < 12 years of age: 0.5 mg.
- If > 12 years of age: 1.0 mg.
Monitor blood glucose every 15 minutes until stable, then repeat 1–2 hourly.
Keep blood glucose between 6 and 8 mmol/L.
REFERRAL
- Recurrent episodes of hypoglycaemia.
DIABETIC NEPHROPATHY
E10.21
DIAGNOSTIC CRITERIA
- Persistent microalbuminuria:
- 3 specimens over a 3–6 month period all show increased albumin:creatinine ratio on a spot urine:
males: >2.5 mg/mmol,
females: >3.5 mg/mmol.
- 3 specimens over a 3–6 month period all show increased albumin:creatinine ratio on a spot urine:
- Screening for microalbuminuria should start from:
- prepubertal children: 5 years post diabetes diagnosis.
- pubertal children: 2 years post diabetes diagnosis.
GENERAL AND SUPPORTIVE MEASURES
- Optimise diabetes control.
- Monitor blood pressure.
MEDICINE TREATMENT
If urinary albumin:creatinine ratio is persistently above reference range for sex:
- ACE inhibitor, e.g.:
- Enalapril, oral, 0.1 mg/kg/dose as a single dose or two divided doses.
- Maximum dose: 0.5 mg/kg or 40 mg/day.
Note: Exclude non-diabetic nephropathy.
Note: Discuss patient with an endocrinologist or nephrologist if there is a poor response to ACE inhibitor and improved glycaemic control.
DYSLIPIDAEMIA
E78.9
DIAGNOSTIC CRITERIA
Refer to Chapter 4: Cardiovascular system - section 4.10 Dyslipidaemia.
GENERAL AND SUPPORTIVE MEASURES
- Optimise diabetes control.
- Refer to a dietician.
- Increase physical activity.
- Members of household who smoke to stop smoking.
MEDICINE TREATMENT
If no improvement in LDL levels after 6 months of exercise and dietary interventions, commence statins (Refer to Chapter 4: Cardiovascular system - section 4.10 Dyslipidaemia).
DIABETES MELLITUS IN ADOLESCENTS
E10
DESCRIPTION
Adolescence is the period between 10 to 19 years of age. The adolescent and the transition should be managed with special planning, i.e.:
- the admission policy of the hospital,
- observing the wishes of the adolescent,
- emotional and physical maturity considerations,
- presence of any co-existing medical, surgical or psychiatric disorder that may be more appropriately managed in the paediatric service.
Aggression and agitation may be features of poorly controlled diabetes.
GENERAL AND SUPPORTIVE MEASURES
Promote:
- normal growth and pubertal development,
- psychological development,
- maintenance of glycaemic control and adherence,
- normal lifestyle,
- avoidance of risk-taking behaviours (smoking, substance abuse),
- sex education.
Adolescents with diabetes may have concomitant behavioural and psychiatric disorders. Anxiety disorders are common in adolescents and should be differentiated from hypoglycaemic and hyperglycaemic episodes.
MEDICINE TREATMENT
Failure of current insulin regimens may be attributed to the endocrine changes of puberty which results in poor glycaemic control.
Insulin resistance occurs during puberty, being maximal in late puberty.
Other causes of poor glycaemic control include family dynamics (e.g. resistance to parental supervision), emotional lability and risk-taking behaviour (e.g. intentionally neglecting to inject and substance abuse).
Normal insulin requirements during puberty:
- 1.0–1.4 units/kg/day.
This may occasionally be higher (up to 2.0 units/kg/day), but as a general rule a higher requirement generally necessitates the search for non-adherence and poor absorption through injections in lipohypertrophy sites.
After puberty, the insulin requirements fall to prepubertal levels.
Failure to reduce insulin requirements in the late adolescent stages may result in excessive weight gain.
DIABETES MELLITUS, TYPE 2
E11
DESCRIPTION
Type 2 diabetes develops when insulin secretion cannot meet the increased demand posed by insulin resistance. Type 2 diabetes may be associated with hyperlipidemia, hypertension, acanthosis nigricans, ovarian hyperandrogenism and non-alcoholic fatty liver disease (features of insulin resistance).
DIAGNOSTIC CRITERIA
Clinical
- Obese or overweight.
- Children with a strong family history of type 2 diabetes, usually in adolescents with BMI >95% without auto-antibodies to islet cells and normal serum C-peptide levels.
- Keto-acidosis is unusual in type 2 diabetes.
- A fasting glucose >7 mmol/L.
- Type 2 diabetics may have minimal symptoms or signs for months or even years before the diagnosis.
Investigations
To confirm diagnosis:
- Symptoms of diabetes.
PLUS - Fasting plasma glucose > 7.0 mmol/L.
OR - Random plasma glucose > 11mmol/L.
OR - No symptoms, but an abnormal 2 hour serum glucose level on the oral glucose tolerance test:
- Ingestion of 1.75 g/kg (maximum 75 g) of glucose dissolved in water.
- Serum glucose > 11 mmol/L 2 hours post ingestion of oral glucose.
GENERAL AND SUPPORTIVE MEASURES
- Lifestyle modification:
Manage patients who are not ill at diagnosis initially with advice on nutrition and exercise, but most will eventually require medicine therapy. - Education on routine blood glucose monitoring. A logbook with all blood glucose reading should be kept. In most cases fasting, prebreakfast measurement and 2-hour postprandial dinner measurement are sufficient.
- Initial medicine treatment is determined by symptoms, severity of hyperglycaemia and presence of ketosis. This should be decided in consultation with a specialist who is experienced in treating these children.
MEDICINE TREATMENT
Refer for initiation of therapy.